Newly Released Annex 1 Looks to Modernize the Manufacture of Sterile Medicinal Products
Are you prepared for the new requirements set forth by Annex1?
The European Medicines Agency (EMA), Pharmaceutical Inspection Co-operation Scheme (PIC/S), and World Health Organization (WHO) has released a new globally harmonized revision of Annex 1: Manufacture of Sterile Medicinal Products. With this extensive update, the worldwide regulatory bodies have sent a strong signal that they want the pharmaceutical industry to embrace modern manufacturing practices when making sterile drugs. Employing these practices can help alleviate drug shortage issues and patient safety concerns through increased product quality and manufacturing efficiency. This relies heavily on the principles of Quality Risk Management (QRM) and encouraging the use of new technologies.

This change in focus can be seen in the requirements for environmental monitoring. The previous version of Annex 1 was often sited as inhibiting the adoption of new monitoring methods. The new revision goes beyond simply removing it as a barrier to outright encouraging it. This can be seen with the statement:
The adoption of suitable alternative monitoring systems such as rapid methods should be considered by manufacturers in order to expedite the detection of microbiological contamination issues and to reduce the risk to product
It is clear that what is acceptable now may no longer be acceptable in the future. Manufacturers will be expected to look to employ new methods if they can improve process understanding and reduce the risk of product contamination.
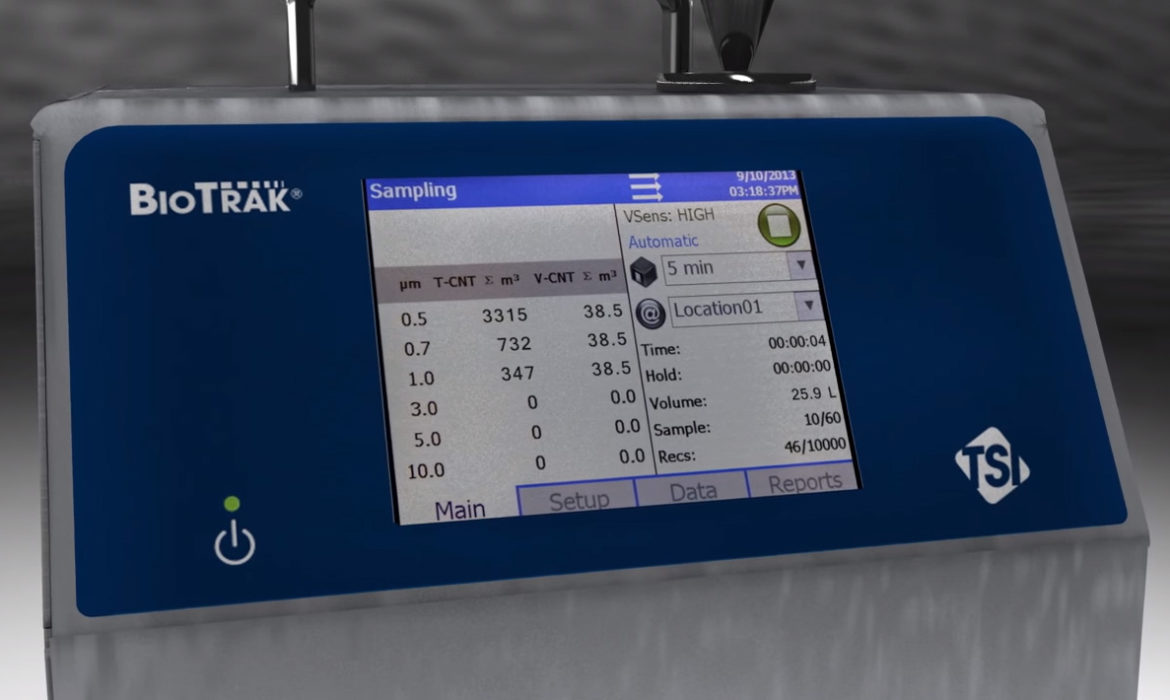
BioTrak Real-Time Viable Particle Counter
Provides real-time counts of total and viable particles in pharmaceutical manufacturing environments to reduce aseptic interventions, improve root-cause investigations, and increase process knowledge.
Truly isolate your aseptic process with the BioTrak® Real-Time Viable Particle Counter (a biofluorescent particle detector).
An alternative and rapid microbiological method (ARMM) for viable air testing, it combines viable particle counting—also called biofluorescent particle counting (BFPC)—with sample capture capability and ISO-compliant total particle counting to offer a complete solution for pharmaceutical environmental monitoring. Superior discrimination between viable and inert particles delivers reliable viable particle data. Time-tested gelatin filter technology efficiently captures sampled microorganisms for subsequent identification.
When combined with TSI FMS Software, it can be used for continuous monitoring of aseptic processes to reduce risk by eliminating environmental monitoring (EM) interventions and improve quality through better process understanding. Automated data collection with the 21 CFR Part 11 capable FMS Software assures the highest level of data integrity.
Beyond the monitoring of aseptic processes, it can also provide value as a standalone instrument. These applications include routine periodic testing, quickly identifying sources of contamination during EM investigations, collecting data to allow for immediate room release, and aiding in the identification and mitigation of risk for improved risk assessments. Enhanced data integrity can be achieved for standalone applications by placing it in Data Integrity Mode and using it in conjunction with the included TSI TrakPro™ Lite Secure Software, Data Integrity Mode is designed per the ALCOA+ principles to meet the demanding regulatory requirements of GMP users of TSI portable particle counting instruments.
Applications that can benefit from using this alternative and rapid microbiologial method (ARMM) for monitoring includes:
- Continuous automated monitoring of aseptic environments
- Continuous automated monitoring in support environments
- Improved root cause investigations
- Room release
- Risk reduction

Meeting Requirements for EU GMP Annex 1 — Features and Benefits
- Detection of airborne viable particles in real-time:
- Fully automated process – no media or manual processing
- Simultaneous detection of both total and viable particles:
- Complies with all requirements of ISO 21501-4
- 1 CFM (28.3 L/min) sample flow rate
- Only requires a single isokinetic probe at the sample site
- In-line particle collection filter
- Can be used as a standalone instrument or fully integrated with FMS for automated continuous monitoring
- Intuitive icon driven touch screen Graphical User Interface
- Stainless steel enclosure and HEPA filtered exhaust