Manufacture of Sterile Products – Annex 1: Particle Counting Considerations
August 25, 2023 is the deadline to comply with the newly published update to EU GMP Annex 1. Having increased from 16 pages to 58, there is obviously a lot for pharmaceutical manufacturing...
Focus on the “Smoke test”
Introduction to the smoke study
Critical manufacturing increasingly frequently requires high quality standards and constant repeatability. For many years now, a multitude of...
Newly Released Annex 1 Looks to Modernize the Manufacture of Sterile Medicinal Products
Are you prepared for the new requirements set forth by Annex1?
The European Medicines Agency (EMA),...
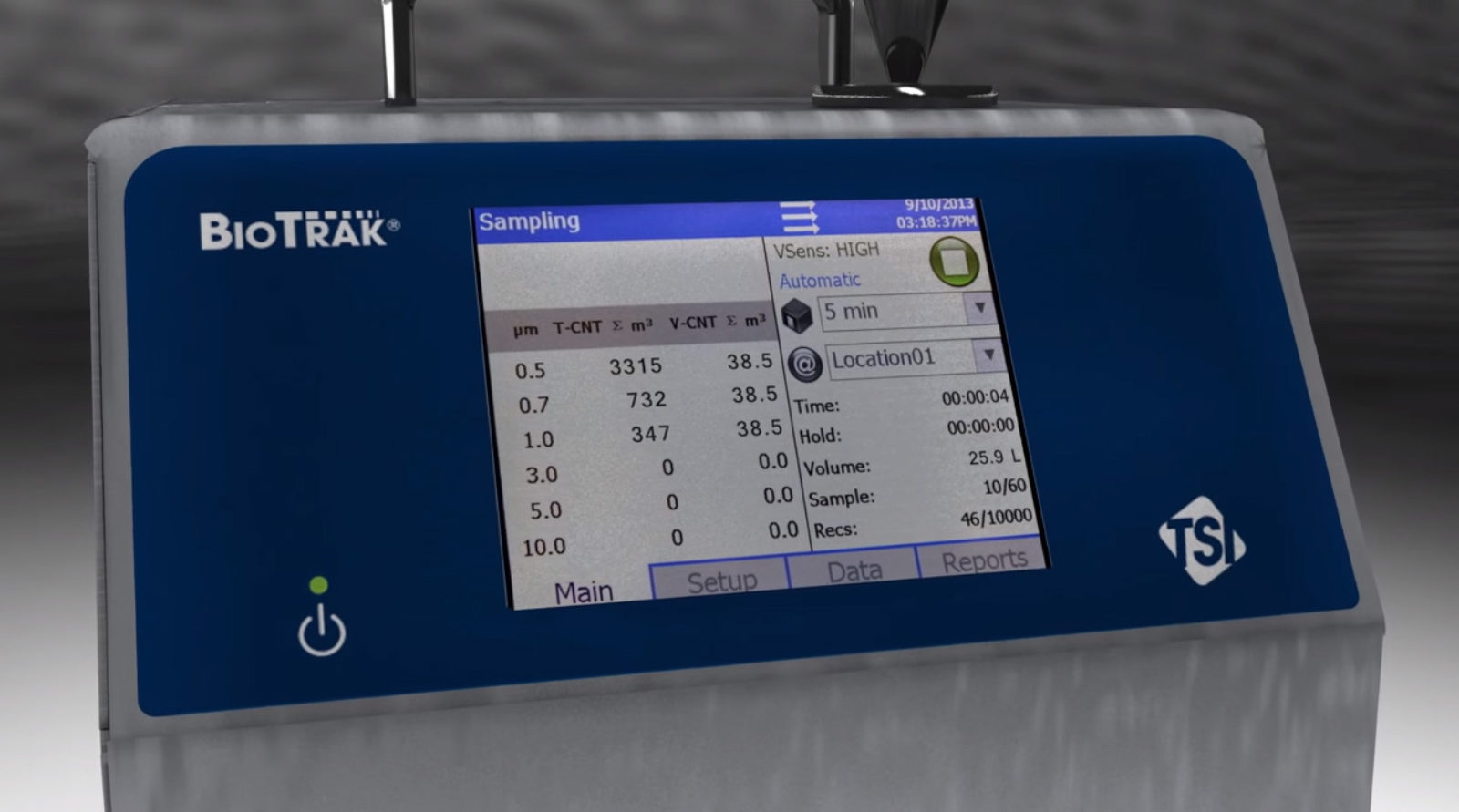
BioTrak Real-Time Viable Particle Counter
Provides real-time counts of total and viable particles in pharmaceutical manufacturing environments to reduce aseptic interventions, improve root-cause investigations, and increase process...